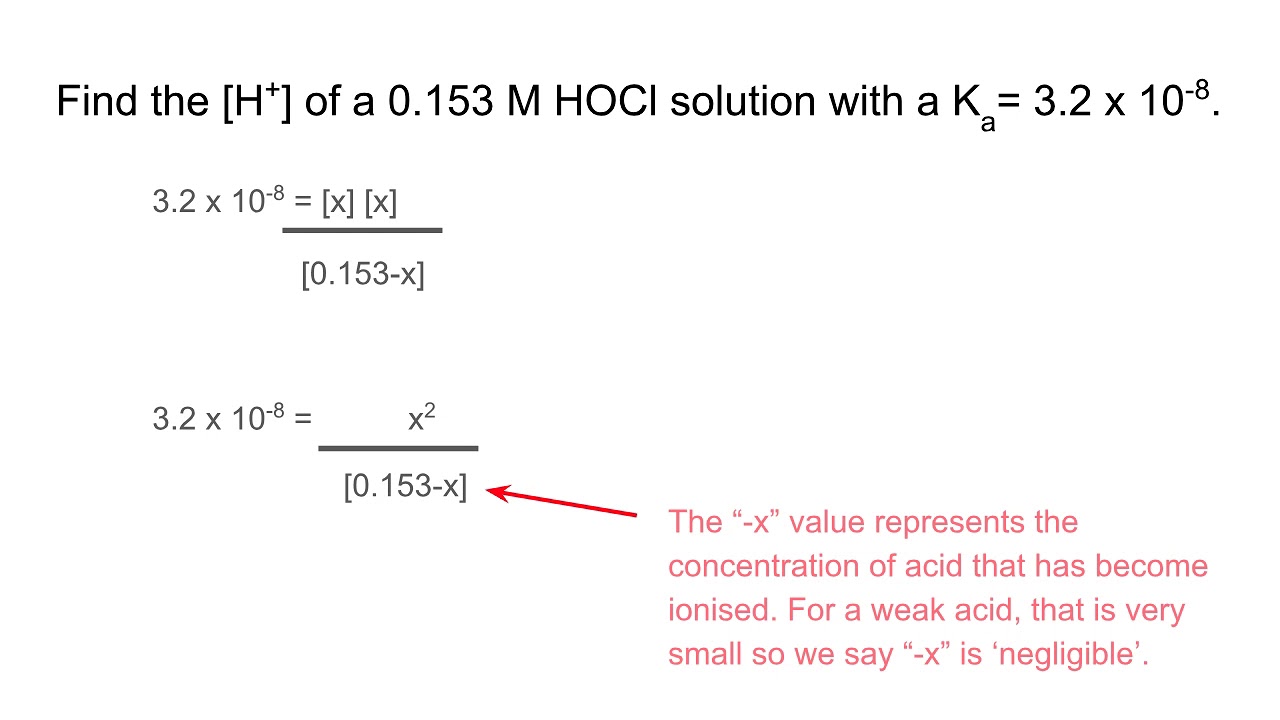
Positive regulation of the enzymatic activity of gastric H+,K+-ATPase by sialylation of its β-subunit - ScienceDirect
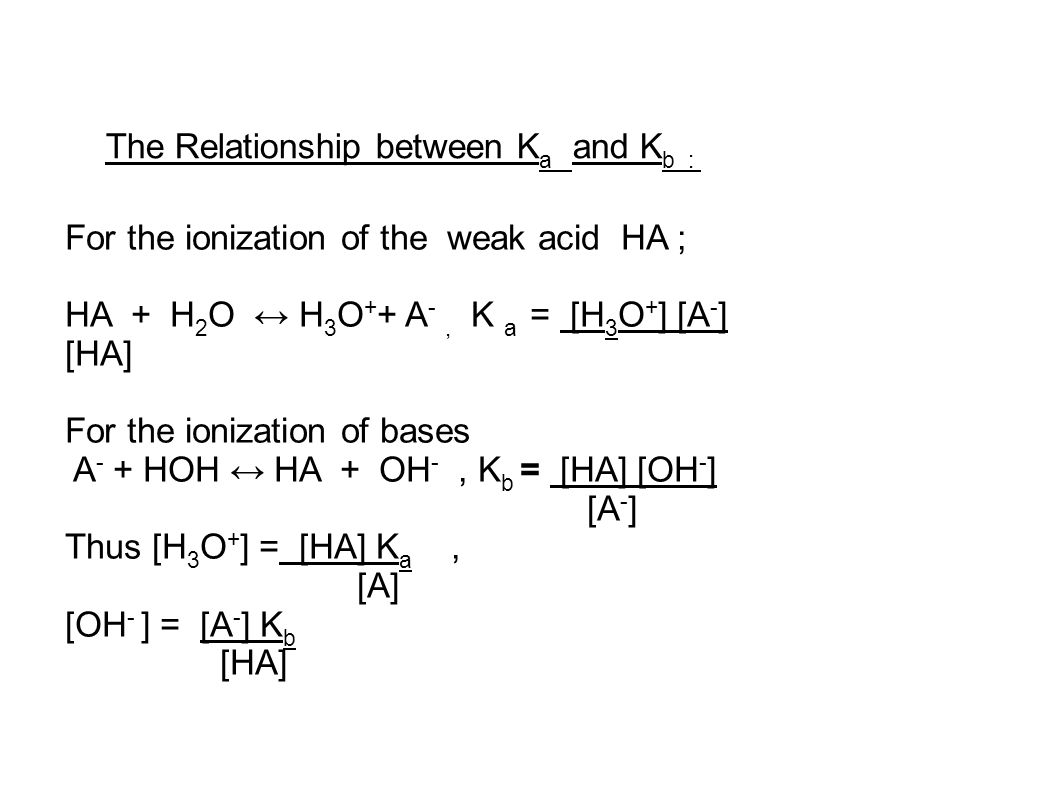
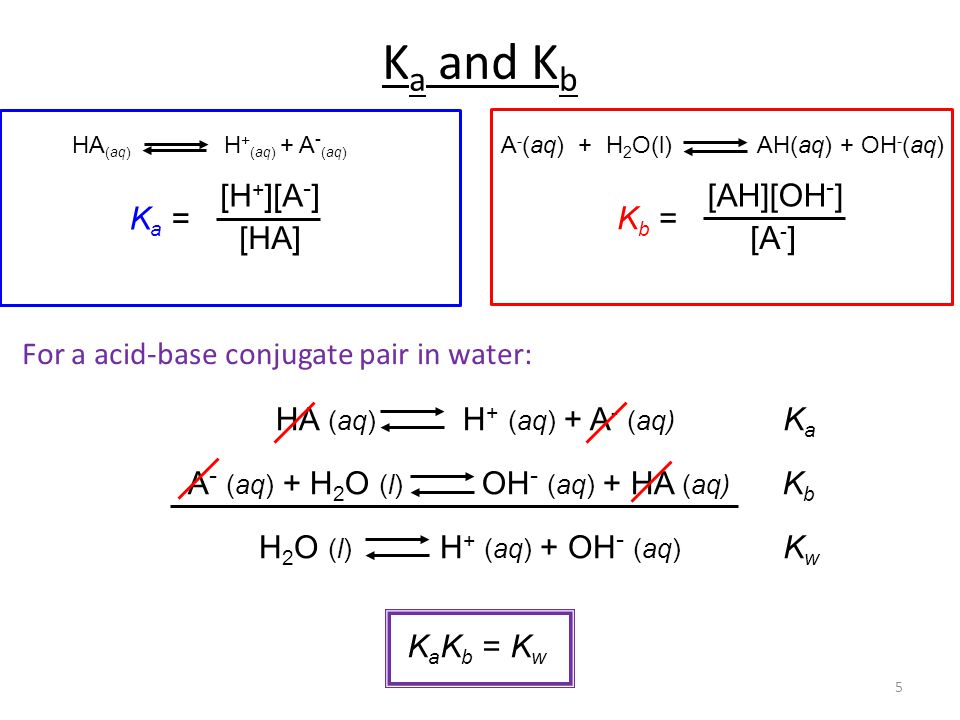
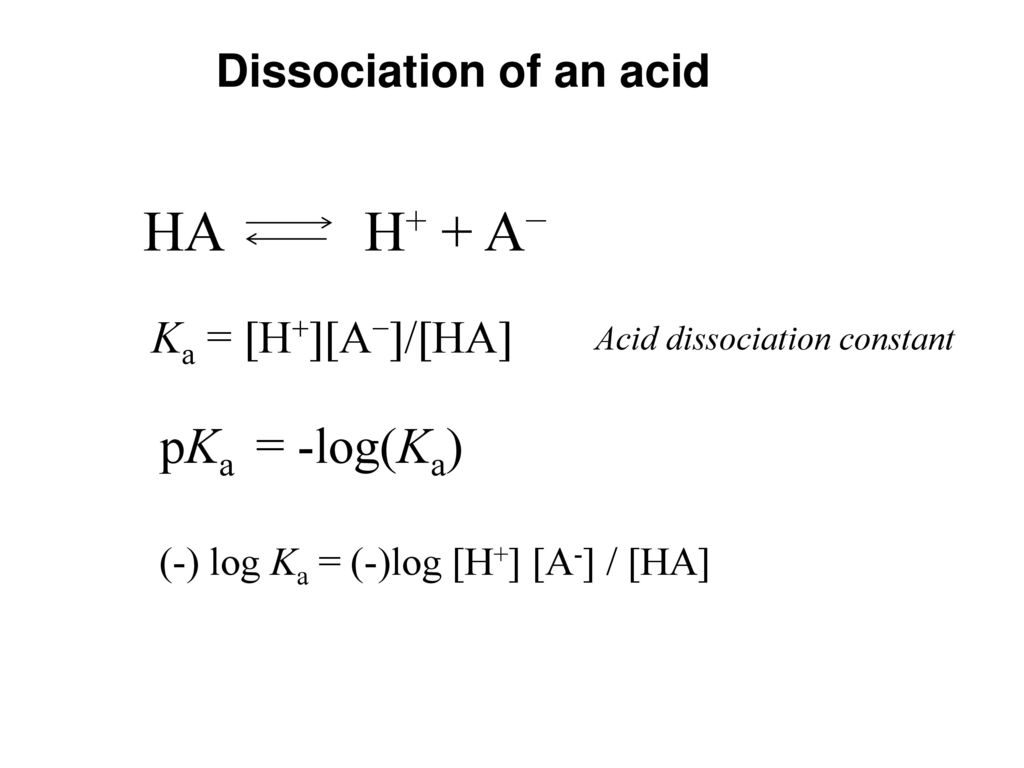
The Relationship between K a and K b : For the ionization of the weak acid HA ; HA + H 2 O ↔ H 3 O + + A -, K
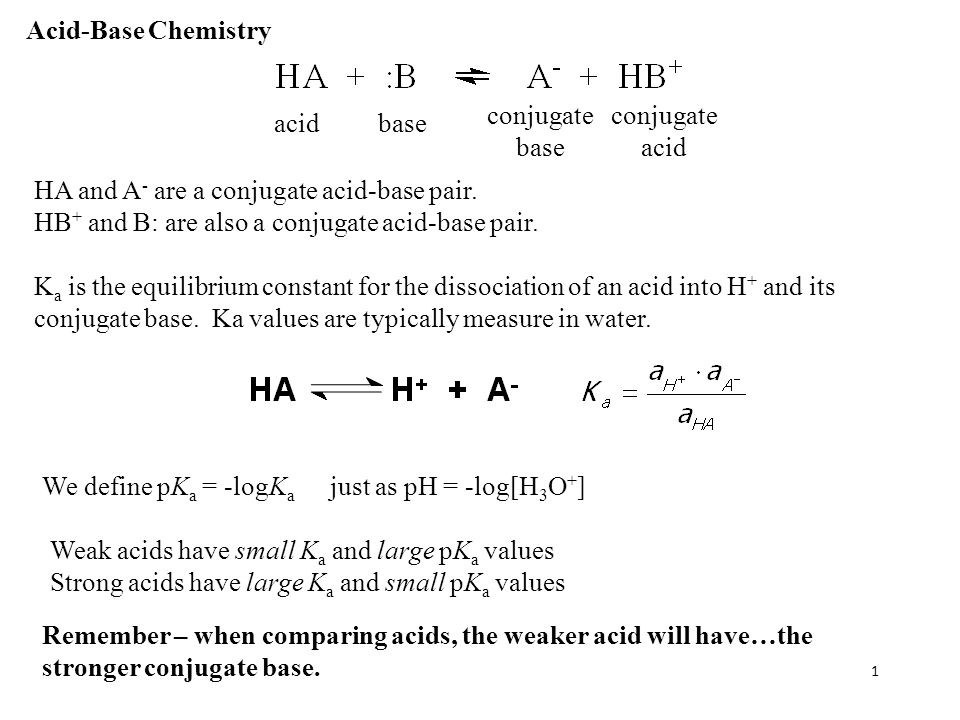
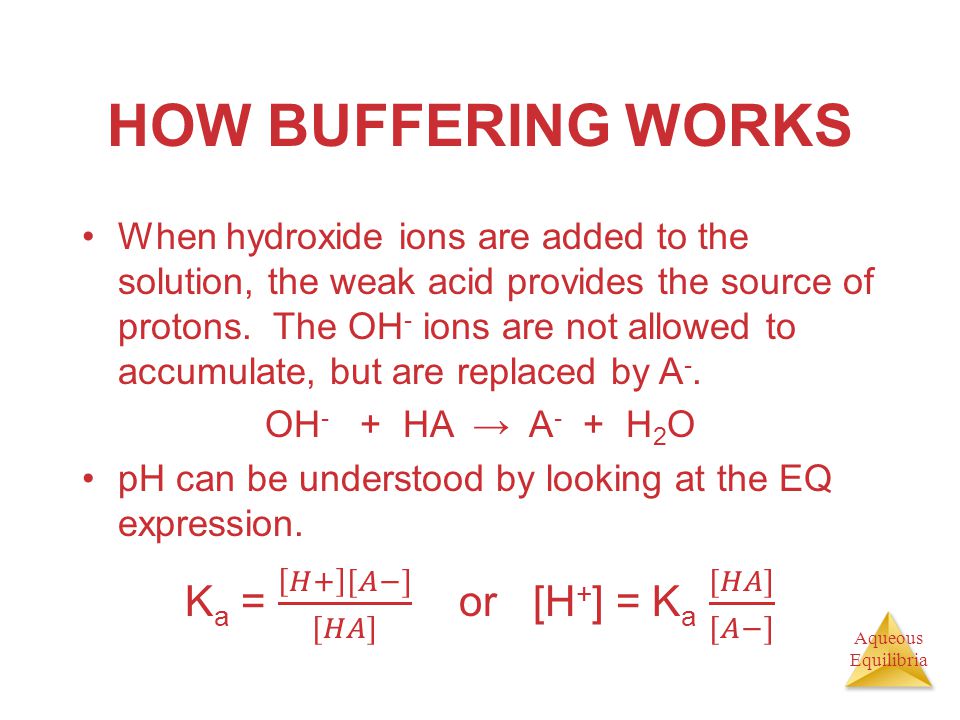
Acid-Base Chemistry K a is the equilibrium constant for the dissociation of an acid into H + and its conjugate base. Ka values are typically measure in. - ppt download

How To Calculate the PH of a Buffer Solution | Equation & Example - Video & Lesson Transcript | Study.com




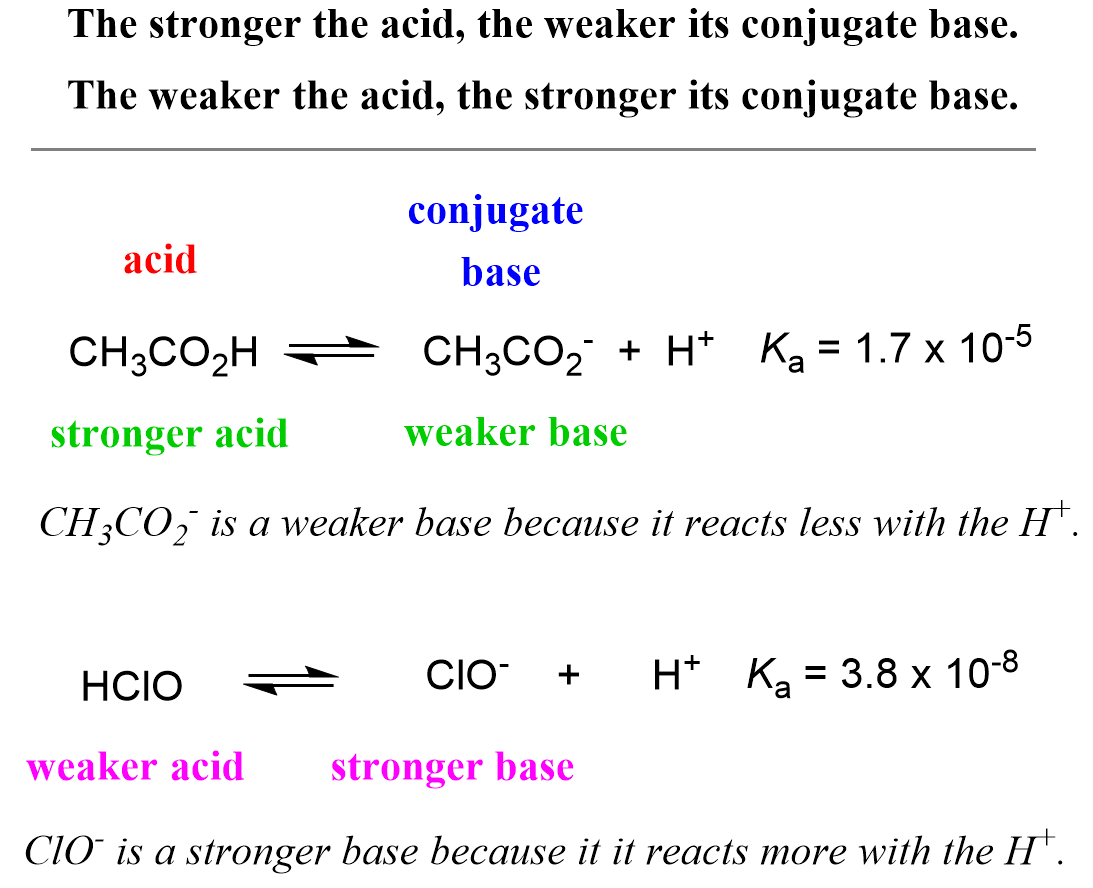
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)
![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)